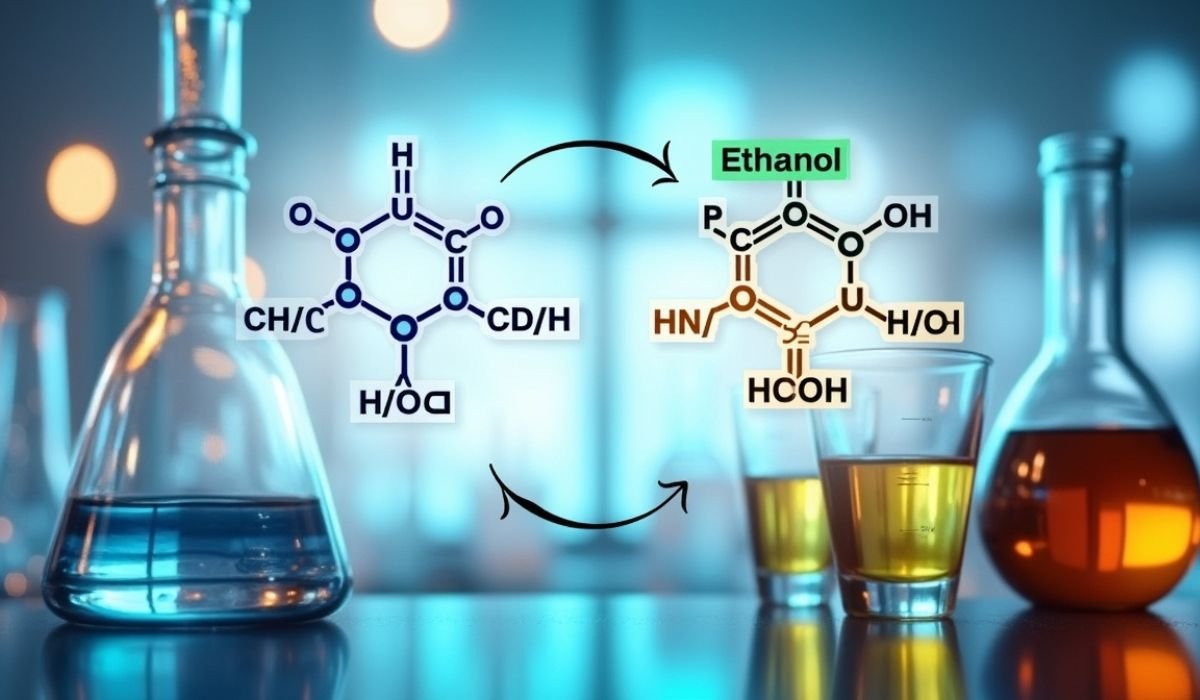
Think of a reaction so precise it turns a simple ester into the tang of your favorite soda or the disinfectant in your medicine cabinet. The combination “HCOOCH CH2 H2O” might look like alphabet soup, but it’s the secret code to a chemical dance called ester hydrolysis. At its core, this process transforms ethyl formate (HCOOCH2CH3) and water into formic acid (HCOOH) and ethanol—a reaction that powers industries from pharmaceuticals to food flavoring. Let’s break down how this unassuming trio of molecules shapes your world.
Why Ester Hydrolysis Is Chemistry’s Unsung Hero
Esters are everywhere: in the scent of pineapples, the smoothness of lotions, and even the fuel additives in your car. Hydrolysis—where water splits these compounds—is like a molecular locksmith, cracking esters into acids and alcohols. Ethyl formate’s hydrolysis is a textbook example:
The Reaction Simplified:
HCOOCH2CH3 (ethyl formate) + H2O → HCOOH (formic acid) + CH3CH2OH (ethanol)
This isn’t just lab magic. It’s a scalable, efficient way to produce chemicals we rely on daily.
How Ethyl Formate Hydrolysis Works: A Step-by-Step Breakdown
Think of hydrolysis as a two-step tango between water and the ester:
- Nucleophilic Attack: Water’s oxygen (the nucleophile) targets the ester’s carbonyl carbon.
- Cleavage: The ester bond breaks, releasing formic acid and ethanol.
Conditions Matter:
- Acidic vs. Basic: Use acid (like H2SO4) for reversible reactions or base (like NaOH) for irreversible saponification.
- Heat: Speeds up the reaction—common in industrial settings.
Real-World Applications: Where You’ll Find This Reaction
Ethyl formate hydrolysis isn’t confined to textbooks. Here’s where it shines:
| Industry | Use Case | Outcome |
|---|---|---|
| Food & Beverage | Flavoring agents (e.g., raspberry) | Formic acid adds tartness; ethanol preserves. |
| Pharmaceuticals | Antiseptic production | Formic acid disinfects; ethanol solubilizes drugs. |
| Textiles | Dye synthesis | Ethanol acts as a solvent for pigments. |
Ethyl Formate vs. Other Esters: What Makes It Unique?
Not all esters hydrolyze equally. Compare:
| Ester | Hydrolysis Products | Key Use |
|---|---|---|
| Ethyl Formate | Formic Acid + Ethanol | Food flavoring, disinfectants |
| Methyl Acetate | Acetic Acid + Methanol | Paint solvents, plastics |
| Isopropyl Palmitate | Palmitic Acid + Isopropanol | Cosmetics, lubricants |
Ethyl formate stands out for its volatility (evaporates easily) and low toxicity, making it ideal for food and pharma.
3 Myths About Ester Hydrolysis—Busted!
- Myth: “Hydrolysis requires extreme conditions.”
Truth: While heat accelerates it, room-temperature hydrolysis occurs slowly (think: ester expiration dates). - Myth: “All esters smell fruity.”
Truth: Ethyl formate has a rum-like aroma, but others (like methyl salicylate) smell minty. - Myth: “Hydrolysis is irreversible.”
Truth: Acidic hydrolysis is reversible; basic (saponification) is not.
FAQs
Q1: Why is ethyl formate hydrolysis important industrially?
It’s cost-effective and yields high-purity formic acid, crucial for preservatives and leather tanning.
Q2: Can I perform this reaction at home?
Not safely—concentrated formic acid is corrosive. Leave it to labs!
Q3: Does hydrolysis destroy the ester’s properties?
Yes—it converts the ester into new compounds with different uses.
Q4: How do catalysts like sulfuric acid help?
They lower activation energy, speeding up the reaction without being consumed.
Q5: Is this reaction eco-friendly?
Depends on disposal methods. Ethanol is biodegradable; formic acid requires careful handling.
Take Action: How to Leverage This Knowledge
- Spot Esters in Ingredients: Look for names ending in “-ate” (e.g., ethyl formate).
- Experiment Safely: Try hydrolysis with vinegar (acetic acid) and baking soda for a DIY demo.
- Stay Curious: Follow companies like BASF or Dow Chemical—they’re ester hydrolysis pioneers.
Final Thought:
Next time you savor a raspberry-flavored treat or disinfect a cut, remember: a tiny ester’s hydrolysis made it possible. Chemistry isn’t just reactions—it’s the invisible hand shaping your life. What other everyday miracles will you uncover?
You May also Like: Wepbound: The 2023 Weight Loss Breakthrough You Need to Know About